
Laura J. Esserman, M.D., M.B.A.
- Professor of Surgery and Radiology
- Division of Surgical Oncology
- Chief, Section of Breast Care Surgery
- Alfred A. de Lorimier Endowed Chair in General Surgery
- Director, UCSF Breast Care Center
Contact Information
Education
1974-77, Harvard University, A.B.
1978-83, Stanford University, M.D.
Residencies
- 1983-85, Stanford University School of Medicine, Resident, General Surgery
- 1988-90, Stanford University School of Medicine, Resident, General Surgery
- 1990-91, Stanford University School of Medicine, Chief Resident, General Surgery
Clinical Expertise
- DCIS (Ductal Carcinoma in situ)
- Inflammatory Breast Cancer
- Invasive Breast Cancer
- LCIS (Lobular Carcinoma in situ)
- Lumpectomy/Partial Mastectomy
- Lymphadenectomy
- Mastectomy
- Recurrent Breast Cancer
- Sentinel Lymph Node Biopsy
- Wire Localization Surgery
Research Interests
- Breast Diseases
- Breast Neoplasms
- Cancer Vaccines
- Genes
- Intraductal Carcinoma
- Male
- Mammary
- Mastectomy
- Noninfiltrating Carcinoma Lobular
- Oncogenes
- Precancerous Conditions
- Segmental
- Tumor Suppressor
- Ultrasonography
- BRCA1 Protein
- BRCA2 Protein
- Infiltrating Duct Carcinoma
Biography
Dr. Laura Esserman, M.D., M.B.A is a surgeon and breast cancer oncology specialist practicing at the UCSF Breast Care Center where she has also held the position of Director since 1996. She co-leads the Breast Oncology Program, the largest of the UCSF Helen Diller Comprehensive Cancer Center's multidisciplinary programs. The program is comprised of 69 faculty members who represent 16 academic specialties and is internationally recognized and well-established with major initiatives in epidemiology, genetics, biology, therapeutics, and clinical cancer care. She is a professor of Surgery & Radiology at UCSF and Associate Director of the UCSF Helen Diller Family Comprehensive Cancer Center where she has founded and led the program in Translational Informatics. As part of this program, her research has focused on bioinformatics, medical and clinical informatics, systems integration, and clinical care delivery.
Dr. Esserman received her Bachelor's degree in History of Science from Harvard University and completed her M.D. at Stanford University. She completed her surgery residency and oncology fellowship at Stanford University Medical Center. After her training, she joined the faculty at Stanford and received a Hartford fellowship to attend Stanford Business School where she received her M.B.A. in 1993. She then joined the faculty at the University of California, San Francisco. She has worked at UCSF to develop interdisciplinary teams of clinicians and researchers to bring the best care to patients and find the best platform to integrate translational research and improve the delivery of breast cancer care.
Dr. Esserman has been a leader in the I-SPY TRIAL collaboration, a National Cancer Institute's (NCI) Center for Bioinformatics and SPORE program. In 2005, she received the NCI SPORE Investigator of the Year Award, an internationally recognized honor and designation. As the Primary Investigator of a Department of Defense-funded Center of Excellence grant, she has also brought together an extraordinary, multidisciplinary group of investigators and health care industry partners to work on critical problems concerning the quality of breast cancer care. Highly respected by her peers, Dr. Esserman was named to the list of U.S. News "America's Top Doctors," a distinction reserved for the top 1% of physicians in the nation for a given specialty.
Dr. Esserman is a prolific writer with numerous publications in peer-reviewed journals covering all aspects of breast health including information systems, immunology, decision making, health policy and the use of imaging. She speaks extensively at public and private forums within the U.S. and internationally. Overall, Dr. Esserman's research and writing tends to focus on the goal of giving patients better access to accurate information so that they can become partners in their health care.
Mentoring Overview
Translational Co-Lead: Dr. Laura Esserman, is internationally recognized for her leadership of the ground-breaking I-SPY2 clinical trial and as an advocate for de-escalation of treatment for DCIS, and the initiation of the virtual preference pragmatic personalized screening trial (WISDOM) seeking to personalize the approach to screening. Her work in breast cancer spans the spectrum from public policy issues to basic science and the impact of both on the delivery of clinical care. Dr. Esserman has mentored 36 post-doctoral or clinical fellows during her career. She developed and runs a post-baccalaureate program that has trained 123 students over the past 15 years, most of whom have gone on to careers in medicine.
Dr. Esserman's broad research interests provide exceptional opportunities for multidisciplinary training across the three themes, through clinical trials and biomarker studies on screening, prevention and treatment of breast cancer, many of which focus on improving healthcare value. Another fundamental and scalable effort is the implementation of systems to integrate care and research.
Research & Funding
- Surgical Oncology Training GrantSponsor: NIH/NCISponsor ID: T32CA251070Funding Period:Jul 2020-Jun 2025Principal Investigator
- Extending the Diversity, Reach, and Generalizability of the WISDOM StudySponsor: NIH/NCISponsor ID: R01CA237533Funding Period:Mar 2020-Feb 2025Principal Investigator
- I-SPY2 +: Evolving the I-SPY 2 TRIAL to include MRI-directed, adaptive sequential treatment to optimize breast cancer outcomesSponsor: NIH/NCISponsor ID: P01CA210961Funding Period:Sep 2017-Aug 2022Principal Investigator
- Elucidating the molecular and contextual basis for IDLE ultralow risk lesions and the tumor immune microenvironment of high risk in situ and invasive breast cancersSponsor: NIH/NCISponsor ID: U01CA196406Funding Period:Sep 2015-Aug 2020Principal Investigator
- Modeling the Impact of Targeted Therapy Based on Breast Cancer SubtypesSponsor: NIH/NCISponsor ID: U01CA187945Funding Period:Sep 2014-Aug 2019Principal Investigator
- Integrated Biomarkers to Characterize Breast Cancer RiskSponsor: NIH/NCISponsor ID: U01CA111234Funding Period:Sep 2004-Jun 2011Principal Investigator
Clinical Trials
- Study ID: NCT04821141Start Date: Jul 2021Estimated Completion Date: Jan 2026Condition(s): Risk Reduction, Breast Cancer
- Study ID: NCT04488081Start Date: Jul 2020Estimated Completion Date: Nov 2022Condition(s): COVID-19
- Study ID: NCT03053193Start Date: Apr 2017Estimated Completion Date: Dec 2030Condition(s): Breast Cancer
- Study ID: NCT02872025Start Date: Dec 2016Estimated Completion Date: Aug 2021Condition(s): Carcinoma, Intraductal, Noninfiltrating
- Study ID: NCT02620852Start Date: Aug 2016Estimated Completion Date: Dec 2020Condition(s): Breast Cancer
- Study ID: NCT01439711Start Date: Feb 2012Estimated Completion Date: Jan 2018Condition(s): Breast Cancer
- Study ID: NCT01042379Start Date: Mar 2010Estimated Completion Date: Dec 2026Condition(s): Breast Cancer, Breast Tumor
- Study ID: NCT00788112Start Date: Jul 2009Estimated Completion Date: Aug 2015Condition(s): Breast Cancer
- Study ID: NCT00416403Start Date: Jul 2006Estimated Completion Date: Jun 2011Condition(s): Breast Cancer
- Study ID: NCT00914017Start Date: Jan 2005Estimated Completion Date: Jan 0001Condition(s): Breast Cancer
- Study ID: NCT01258296Start Date: Feb 2003Estimated Completion Date: Aug 2007Condition(s): Postoperative Pain
- Study ID: NCT00429988Start Date: Aug 2002Estimated Completion Date: Jun 2004Condition(s): Breast Cancer
- Study ID: NCT00033397Start Date: Feb 2002Estimated Completion Date: May 2012Condition(s): Breast Cancer
Publications
MOST RECENT PUBLICATIONS FROM A TOTAL OF 423
- Umashankar S, Basu A, Esserman L, Van't Veer L, Melisko ME. Concordance between patient-reported and physician-documented comorbidities and symptoms among Stage 4 breast cancer patients. Cancer Med. 2023 Oct 30. View in PubMed
- Rothschild HT, Clelland EN, Abel MK, Chien AJ, Shui AM, Esserman L, Khan SA, Mukhtar RA. The impact of histologic subtype on primary site surgery in the management of metastatic lobular versus ductal breast cancer: a population based study from the National Cancer Database (NCDB). Breast Cancer Res Treat. 2023 Oct 13. View in PubMed
- Boughey JC, Yu H, Dugan CL, Piltin MA, Postlewait L, Son JD, Edmiston KK, Godellas C, Lee MC, Carr MJ, Tonneson JE, Crown A, Lancaster RB, Woriax HE, Ewing CA, Chau HS, Patterson AK, Wong JM, Alvarado MD, Yang RL, Chan TW, Sheade JB, Ahrendt GM, Larson KE, Switalla K, Tuttle TM, Tchou JC, Rao R, Tamirisa N, Singh P, Gould RE, Terando A, Sauder C, Hewitt K, Chiba A, Esserman LJ, Mukhtar R. ASO Visual Abstract: Changes in Surgical Management of the Axilla Over 11 Years-Report on Over 1500 Breast Cancer Patients Treated with Neoadjuvant Chemotherapy on the Prospective I-SPY2 Trial. Ann Surg Oncol. 2023 10; 30(11):6411-6412. View in PubMed
- Rothschild HT, Clelland EN, Mujir F, Record H, Wong J, Esserman LJ, Alvarado M, Ewing C, Mukhtar RA. ASO Visual Abstract: Predictors of Early Versus Late Recurrence in Invasive Lobular Carcinoma of the Breast: Impact of Local and Systemic Therapy. Ann Surg Oncol. 2023 Oct; 30(10):6007. View in PubMed
- Ho KKY, Kaiser UB, Chanson P, Gadelha M, Wass J, Nieman L, Little A, Aghi MK, Raetzman L, Post K, Raverot G, Borowsky AD, Erickson D, Castaño JP, Laws ER, Zatelli MC, Sisco J, Esserman L, Yuen KCJ, Reincke M, Melmed S. Pituitary adenoma or neuroendocrine tumour: the need for an integrated prognostic classification. Nat Rev Endocrinol. 2023 Nov; 19(11):671-678. View in PubMed
- View All Publications
In the News
- Susan G. Komen - October 01, 2020Susan G. Komen®, the world's leading breast cancer organization, recognized two widely respected and innovative breast cancer researchers this week, announcing that Donald McDonnell, Ph.D., and Laura Esserman, M.D., have been selected as this year's recipients of the Brinker Awards – Komen's highest scientific honor. This year's Brinker Award for Scientific Distinction in Clinical Research will be presented to Laura Esserman, M.D., M.B.A., Director, Carol Franc Buck Breast Care Center, Alfred [...]

- UCSF Breastcare Program - Richard Barg - July 15, 2020Laura J. Esserman, MD, MBA and her research team have been awarded a $9.1M NIH R01 grant from the National Cancer Institue (NCI). The focus of the grant is to enable expansion of The WISDOM Study to new recruitment sites/partnerships including Louisiana State University, the University of Chicago, the University of Alabama, and a women's health clinical care network in Florida. This will advance the goal of increasing diversity in the study, provide the benefits of the study to underserved [...]

- UCSF Department of Surgery - July 13, 2020The UCSF Department of Surgery has just been awarded a paradigm-shifting NIH T-32 training grant for Surgical Oncology. The goal of this new innovative program is to train future leaders in surgery and oncology to be change agents in driving science and value-based medicine by giving them a foundation in translational science, regulatory science and implementation science. Training in these areas will further help move great ideas from bench to bedside. Improving care in the next decade [...]

- Time.com - Laura J. Esserman, M.D., MBA - April 29, 2020"Getting the news that you have cancer is overwhelming and frightening. The COVID-19 crisis adds another layer of anxiety. But know this: you can protect yourself from COVID-19 without compromising your cancer treatment. Don't panic. In the vast majority of cases, a diagnosis of cancer is not an emergency even though it feels like one. There is time to learn about your options and sort out what is right for you. For now, there will be changes to how we do things. Some of the changes will feel [...]

- Quantum Leap Healthcare Collaborative (QLHC) - PR Newswire - April 29, 2020"Quantum Leap Healthcare Collaborative (QLHC), a non-profit organization focused on uniting patient care and research, and sponsor of the adaptive platform I-SPY 2 Trial™, announced today a unique pre-competitive consortium created for an I-SPY COVID Trial to rapidly identify therapies to treat acute respiratory distress syndrome (ARDS) in critically ill COVID-19 patients. Approximately 10-15% of patients infected with SARS-CoV2 develop a severe acute respiratory illness, and nearly 70% of [...]

- UCSF Breastcare Program - Richard Barg - December 16, 2019Laura J. Esserman, MD, MBA has been awarded a $760K FDA Grant through the UCSF-Stanford Center of Excellence in Regulatory Science and Innovation (CERSI). CERSI is a joint undertaking among the UCSF School of Pharmacy and School of Medicine, the Stanford University School of Engineering and School of Medicine and the U.S. Food and Drug Administration (FDA), the first regulatory science and innovation center on the West Coast. The grant funds Phase 2 of the "OneSource" Project, which is [...]

- Fortune.com - Sy Mukherjee - November 21, 2019Dr. Laura Esserman is on a mission to bring the blockchain to health care. I can already hear the audible groans and see the general eye-rolling—I get it. There have been plenty of bold claims when it comes to blockchain, the decentralized ledger technology, in pretty much every sector from food to medicine. So far, the sizzle to steak ratio has been out of whack, and skepticism is integral to good science. But Esserman, a renowned surgeon and director of the UCSF Carol Franc Buck Breast Care [...]
- TIME.com - Mandy Oaklander - October 17, 2019Behind any landmark cure is years of medical research. But the old goal of research — to find a one-size-fits-all treatment for a disease, based on a set of standard protocols — must radically change to further and diversify advances in the field, experts argued at the TIME 100 Health Summit on Thursday. "We're all in it, at the end of the day, for our patients," said Dr. Laura Esserman, professor of surgery at the University of California, San Francisco School of Medicine. "We want to get [...]

- UCSF Breastcare Program - Richard Barg - August 25, 2018Laura J. Esserman, MD, MBA was selected as the 2018 OncLive® "Giant of Cancer Care" in Cancer Diagnostics. Dr. Esserman is professor of Surgery and Radiology at UCSF and director of the UCSF Carol Franc Buck Breast Care Center. She was chosen by an elite committee of her peers based on her long history of outstanding work in the field as noted in the announcement: Dr. Esserman led efforts to address harms of screening including overdiagnosis, and proposed tools including the development of [...]

- UCSF Department of Surgery - Richard Barg - March 27, 2018The UCSF Department of Surgery now ranks No. 3 nationally in NIH research funding among all academic surgery programs, a stunning rise from 6th the last time the survey was done. Three Department of Surgery faculty also ranked in the top 20 of all NIH-funded principal investigators nationally in the category of surgery. The highly respected Blue Ridge Institute for Medical Research utilized data from the Research Portfolio Online Reporting Tool (RePORT) to survey U.S. medical schools for their [...]

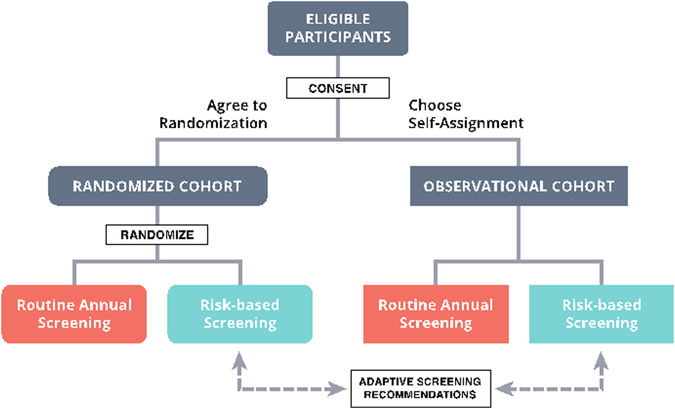
- UCSF Breastcare Surgery - Richard Barg - February 28, 2018Renowned breast surgeon Laura J. Esserman, MD, MBA gave a captivating talk about her richly textured life and innovative research at a Staff Engagement Event hosted by the Department of Surgery on February 9th at UCSF Mount Zion. The research portion of her talk focused on two landmark clinical trials she serves as principal investigator on. The first, the WISDOM Study (Women Informed to Screen Depending on Measures of Risk (WISDOM), is a pragmatic, adaptive, randomized clinical trial [...]

- UCSF Breastcare Surgery - Richard Barg - February 27, 2018An article commentary recently published in NPJ Breast Cancer, a Nature Research journal, discusses the WISDOM Study (Women Informed to Screen Depending on Measures of Risk (WISDOM), a groundbreaking clinical trial recruiting 100,000 women from throughout California that will compare annual breast cancer screening with personalized risk-based breast cancer screening. The study is led by Laura J. Esserman, MD, MBA (pictured), professor of surgery and director of the Carol Franc Buck Breast Care [...]
- UCSF Breastcare Surgery - Richard Barg - July 10, 2017Laura Esserman, M.D., MBA, Professor of Surgery and Radiology at UCSF, and Director of the UCSF Carol Franc Buck Breast Care Center, was recently interviewed by NPR about a study she led utilizing MammaPrint, a diagnostic assay (test), which appears to precisely distinguish between "ultralow-risk" breast tumors and more aggressive ones. This test could inform the decision to whether to recommend adjuvant (post-surgical) therapy, potentially sparing patients from the toxic effects of [...]

- UCSF Breastcare Program - Richard Barg - March 14, 2017UCSF News reports on the results of a new multi-center study showing that scalp cooling can mitigate one of most devastating side effects of systemic chemotherapy, hair loss, in a significant number of breast cancer patients. The results were reported on in JAMA. Hope S. Rugo, MD , was the lead author and Principal Investigator on the study. Michelle E. Melisko, MD, and Laura Esserman, MD (pictured), of UCSF were among the coauthors of the study. Scalp cooling can lessen some [...]

- UCSF Breastcare Program - May 18, 2016UCSF News reports on Time magazine's selection of Laura Esserman, MD, MBA, to the 2016 TIME 100, the magazine's annual list of the 100 most influential people in the world. Dr. Esserman is Professor of Surgery and Radiology, and Director of the Carol Franc Buck Breast Care Center at UCSF. Time magazine has named internationally renowned breast cancer oncologist Laura Esserman, MD, MBA, to the 2016 TIME 100, the magazine's annual list of the 100 most influential people in the [...]

- UCSF Breastcare Surgery - September 30, 2015The NY Times profiled UCSF breast cancer surgeon Dr. Laura Esserman and her challenge to the conventional wisdom surrounding breast cancer screening. Esserman argues that most patients will not benefit from early detection of ductal carcinoma in situ (DCIS) lesions and further that the ominous word "carcinoma" be dropped from the medical term and the condition renamed "indolent lesions of epithelial origin," or IDLEs. Late one afternoon this summer, Dr. Laura J. Esserman, a breast [...]

- UCSF Breastcare Surgery - March 26, 2015UCSF News reports on a new study, led by breast cancer researcher Laura Esserman, MD, MBA, that will compare a personalized approach to breast cancer screening with annual mammograms. A research team at UC San Francisco has won a five-year award of $14.1 million from the Patient-Centered Outcomes Research Institute (PCORI) to investigate whether a personalized approach to breast cancer screening is as safe and effective as annual mammograms. The project, called the WISDOM study, will be [...]

- Kaiser Health News (KHN) - Anna Gorman - March 19, 2015The "shared decision-making" model fosters a higher level of collaboration between doctors and the people they treat. Rose Gutierrez has a big decision to make. Gutierrez, who was diagnosed with breast cancer last spring, had surgery and 10 weeks of chemotherapy. But the cancer is still there. Now Jasmine Wong (pictured right), a surgeon at the University of California, San Francisco, is explaining the choices—Gutierrez can either have another lumpectomy followed by radiation, or she can get [...]

- UCSF Breastcare Surgery - April 07, 2014UCSF News reports on new results showing the promise and potential of I-SPY 2 Trial, personalized medicine study designed to identify breast cancer patients most likely to benefit from an array experimental drugs. In an innovative clinical trial led by UC San Francisco, the experimental drug neratinib along with standard chemotherapy was found to be a beneficial treatment for some women with newly diagnosed, high-risk breast cancer. Additionally, researchers learned that an algorithm used [...]

- UCSF Breastcare Surgery - July 23, 2013UCSF News reports on the recommendations of an NCI scientific panel chaired by Laura Esserman, MD, MBA, Professor of Surgery and Radiology and Director, UCSF Carol Franc Buck Breast Care Center, to reduce overdiagnosis and overtreatment. To address the growing problem of people being overdiagnosed and overtreated for cancer, a group of scientists convened by the National Cancer Institute and chaired by a UC San Francisco breast cancer expert is proposing a major update of the way the nation [...]

- UCSF Breastcare Surgery - July 20, 2009The San Francisco Chronicle recently profiled UCSF breastcare surgeon Dr. Laura Esserman, There is no starched white lab coat for Dr. Laura Esserman. Instead, the tall and striking breast cancer researcher, surgeon and visionary favors colorful clothes, high heels and, on a recent day, metallic blue nail polish. Before she operates, just as the general anesthetic is being administered, Esserman sings the patient's requested song. A talented musician with a voice for opera, Esserman [...]
